
CarrierAdvance is an innovative genetic test that allows identification of couples at risk of transmitting the most common genetic diseases to their children, by detecting DNA mutations of which one or both parents may be unwitting carriers.

Each person is born with genetic characteristics that differentiate him/her
from others and that make this person unique. While most of the differences in the DNA sequence between different
people is harmless, some changes, kown as "mutations", can alter genomic
functionality and make that person a carrier of a specific genetic disease,
transmissible to their children.
In most cases, carriers of genetic diseases are healthy individuals and show
no symptoms, have no known family history of the disease, and are unaware
that they are at risk of passing this DNA "error" to their children.
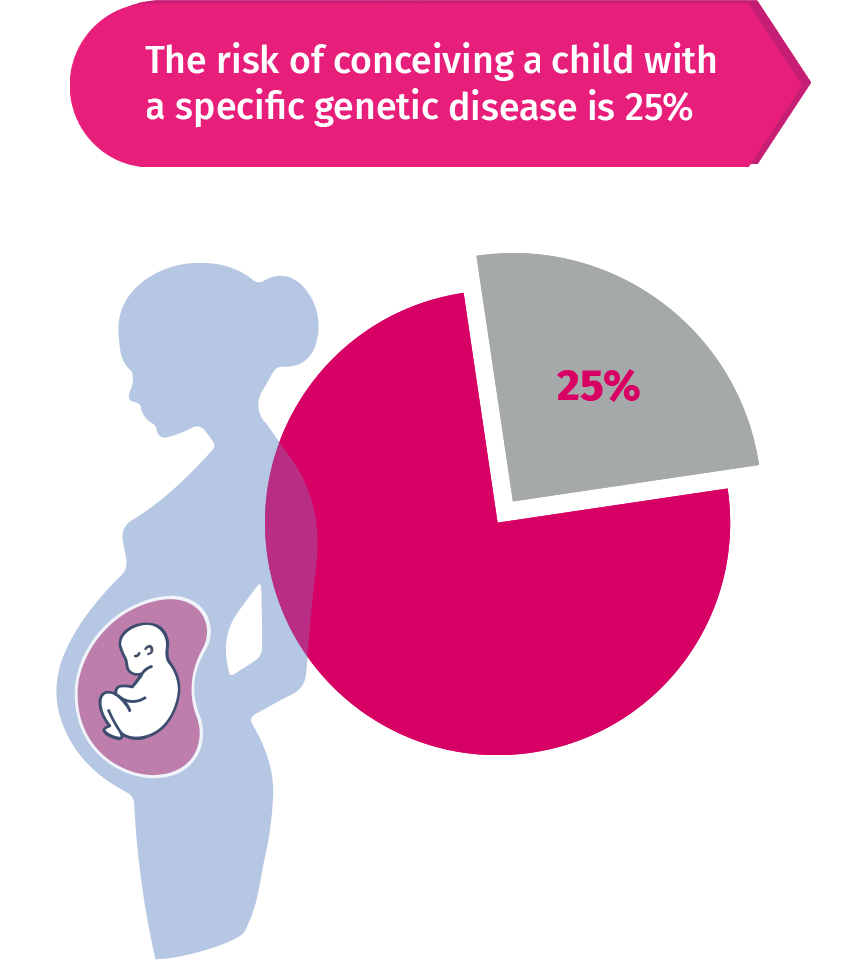
If both partners of the couple carry mutations in the same gene with autosomal
recessive inheritance, there will be an increased risk (25%) of conceiving a
child with that specific disease. If a woman is a carrier of a X-linked disorder,
50% of male children will be at risk of having that specific genetic disease.
INDICATION FOR TESTING

Carrier Advance is intended to be used as a family planning tool, allowing patients to be tested individually or with their reproductive partner for their risk of having children with various genetic conditions.

For patients who are pursuing pregnancy with assisted reproductive technologies.

Couples requiring gamete donation, in order to select the most appropriate donor for each recipient (i.e. a donor that doesn’t carry the same mutation as the member of the couple who will provide the gametes), minimizing the reproductive risk.

Anyone who wants to know if they are carrier of any condition included in the panel.

Individuals with a family history of a genetic disease, who are therefore at higher risk of being carriers for those diseases.
3 LEVELS OF SCREENING


carrieradvance
focus
30 Genes
31 recessive and X-linked genetic disorders, including the most common in the European population

carrieradvance
plus
925 Genes
1467 recessive and X-linked genetic
disorders, including the most common
in the European population and
those recommended by prestigious
international societies, such as ACMG
and ACOG

carrieradvance
exome
4000+ Genes
Clinical exome sequencing, including
5000+ recessive and X-linked genetic
disorders, compatible with the majority
of carrier screening test available in the
market
A GROUNDBREAKING TECHNOLOGY COUPLED WITH AN ADVANCED BIOINFORMATIC ANALYSIS TO DELIVER GREATER ACCURACY

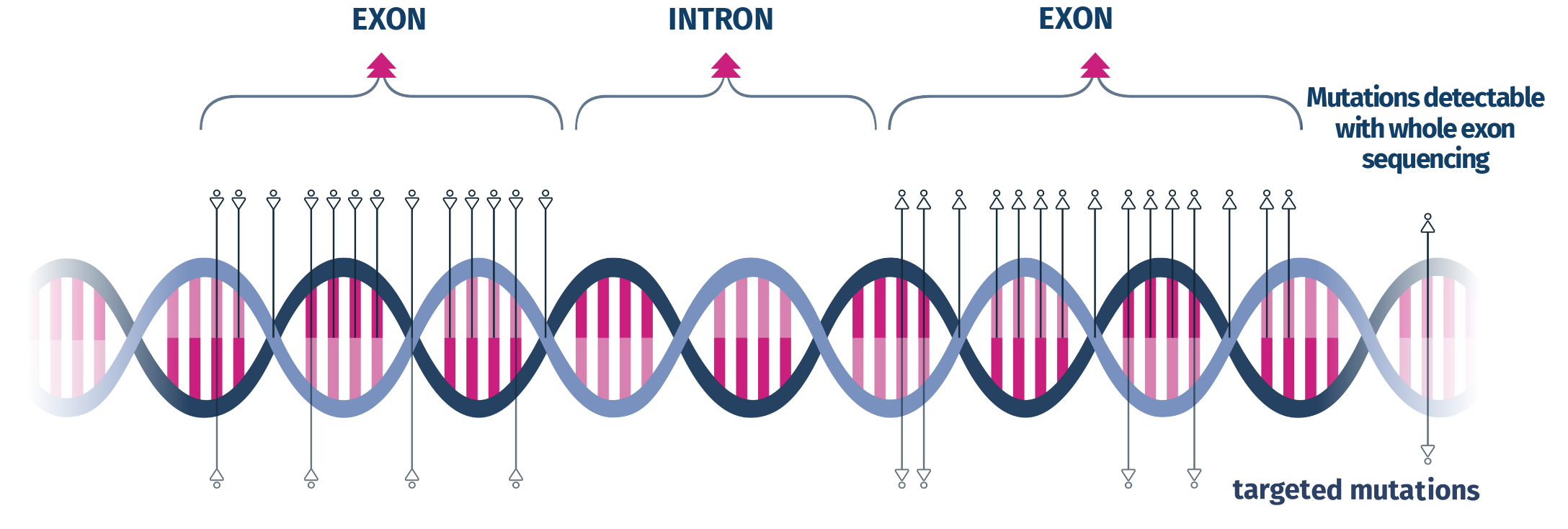
CarrierAdvance uses a state-of-the-art technological process, named Next Generation Sequencing (NGS). Unlike other carrier screening tests using targeted sequencing, CarrierAdvance is carried out performing full exon sequencing of all the genes included in the panel, which allows a more comprehensive assessment of each gene and related diseases.

Type of samples
- Buccal swab
- Peripheral blood
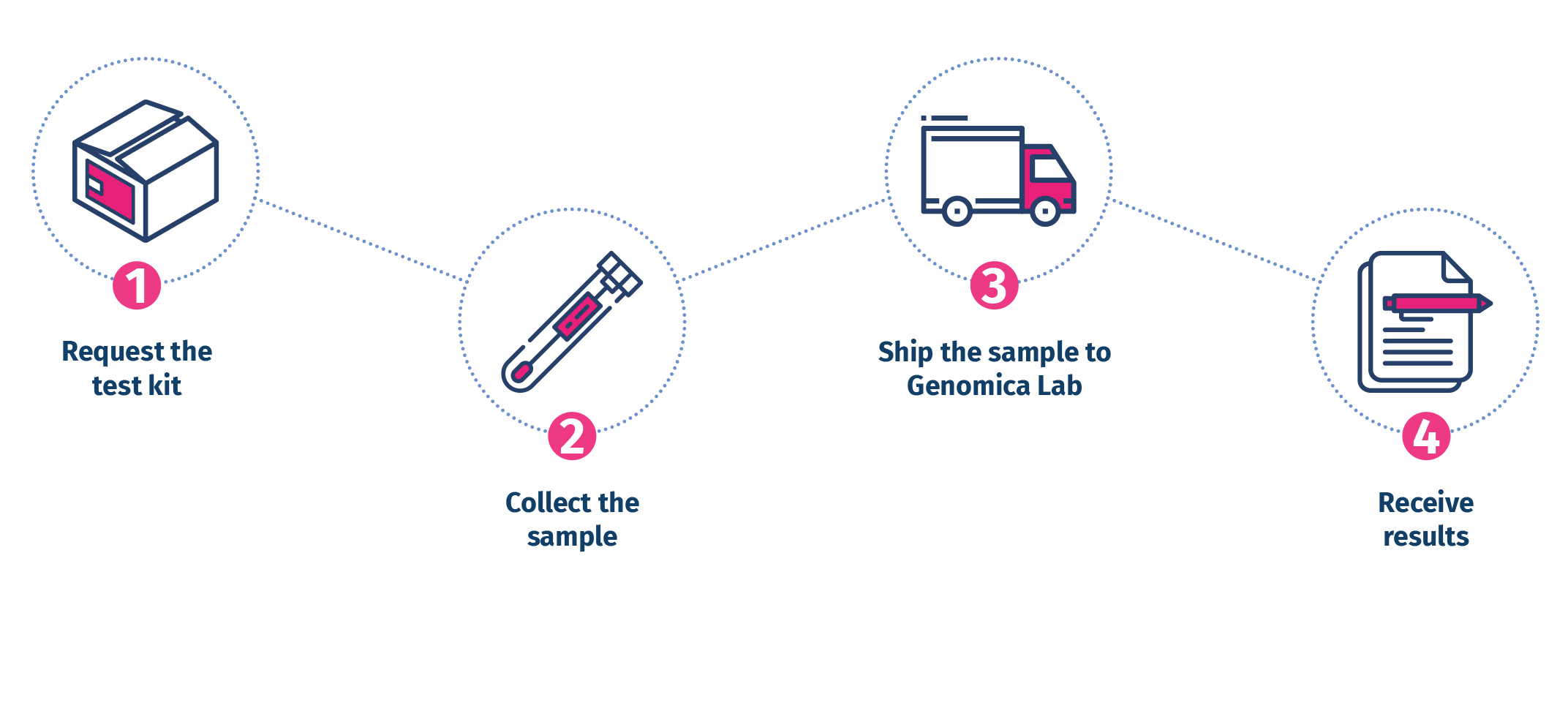
4 easy steps

TEST RESULTS



NEGATIVE
No mutations identified: this test result indicates that not known or likely pathogenic mutations have been detected in the targeted genes screened. A single test cannot always detect all possible genetic changes. Hence, a negative test results do not completely rule out the the possibility of carrying a mutation located in a region of the genome not investigated by the test.

POSITIVE
Identification of one or more mutations: this test result indicates that one or more mutations have been detected in the targeted genes screened, identifying the patient as a carrier. Mutations reported in a POSITIVE CarrierAdvance test may be classified under the following prognosis categories: Known pathogenic: clinical relevant mutations causing well-established syndromes; Likely pathogenic: variants that are likely clinical relevant and may cause well-established syndromes. Classification follows the recommendations of the international reference guidelines. 1

UNCERTAIN CLINICAL RESULT
Variants of uncertain clinical significance (VUS): this test result indicates that one or more variants of uncertain clinical significance have been detected in the targeted genes screened. These are variants with insufficient evidence available for unequivocal determination of clinical significance. VOUS will only be reported if both partners perform the test and one of them is found to be carrier of a known pathogenic mutation.
1Richards et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine. 2015;17(5):405-423.

Turnaround time


Over 100.000
genetic tests/year

Test performed in Italy
(Rome or Milan)

20+ years experience in prenatal molecular diagnostics

Fast
TAT

Dedicated
R&D team

Laboratories with groundbreaking technologies and high quality standards

Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks

International
Partnership

Over 100.000 genetic tests/year

Test performed in Italy
(Rome or Milan)

20+ years experience
in prenatal molecular diagnostics

Fast TAT
3 days

Dedicated
R&D team

Laboratories with groundbreaking technologies and high quality standards

Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks

International
Partnership
INFORMATION REQUEST FOR CARRIERADVANCE TEST
Fill out this form for a free consultation.
One of our professionals will contact you, free of charge and without obligation, to provide you with all the information you need.



 Italiano
Italiano English
English
